[재외 연구자 소개] 신재원 박사 (University of Illinois College of Medicine, Chicago, IL)
관리자
view : 1099

재외 연구자 소개

- Jae-Won Shin, Ph.D.
University of Illinois College of Medicine, Chicago, IL
- 1. Title
-
Shin et al., Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014 Jan 2; 14(1): 81-93.
- 2. Background
-
The human body produces billions of blood cells daily. This critical process requires hematopoietic stem and progenitor cells (HSPCs). For HSPCs to produce blood, they not only have to differentiate into all types of blood cells, but also maintain this capability throughout life by renewing themselves ('self-renewal'). Increasing evidence suggests that the balance between differentiation and self-renewal may occur during stem cell division. When stem cell division is 'symmetric', two daughter cells with similar regenerative potential are produced, and this leads to stem cell expansion. When stem cell division is 'asymmetric', one daughter cell maintains stem cell properties, while the other daughter cell becomes differentiated. While cell division is well understood, what regulates the division of stem cells remains to be elucidated. Cell division is a highly biophysical process and generally requires contractile forces generated by myosin-II to split daughter cells. In addition to this cell-intrinsic role, cells require contractile forces to sense extracellular biophysical signals, including fluid shear and matrix rigidity. However, it remains unclear how contractile forces regulate self-renewal and differentiation during stem cell division. This review highlights some of the key findings from this investigation.
- 3. Results
- 1) Myosin-II isoform switching in human adult hematopoiesis
-
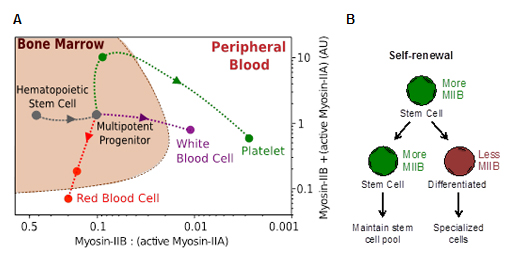
Figure 1. Two-component lineage trajectories of myosin-II isoform states in hematopoiesis. (A) Myosin-II map in adult hematopoiesis. (B) Hypothesized role of myosin-IIB in asymmetric division.
There are three known isoforms of myosin-II in mammals, A, B, and C. Blood cells express A and B isoforms. To initiate this study, protein expression levels of both isoforms were evaluated by using intracellular flow cytometry. The values from these experiments were then converted to absolute ratios and protein quantity by using parameters derived from sample standards measured against label-free quantitative mass spectrometry. The results demonstrate that the B isoform is downregulated during differentiation of nucleated cells up to ~100 fold in terms of the B:A ratio (Fig. 1A). Erythroid lineages undergo reduction in total myosin-II expression, while megakaryocyte lineages accumulate myosin-II but platelets retain predominantly myosin-IIA. Based on these observations, it was then hypothesized that myosin-IIB is asymmetrically segregated to one daughter cell during division (Fig. 1B). Because the actin-myosin complex is sensitive to external forces, effects of locally applied forces on subcellular distribution of myosin-IIB were investigated.
2) Myosin-IIB but not myosin-IIA is mechanosensitive-
After overexpressing fluorescently tagged myosin-IIB and IIA, cells were subjected to micropipette aspiration under a constant pressure (<1 kPa) (Fig. 2A). Myosin-IIB is preferentially localized to the site where negative pressure is applied, while myosin-IIA shows more global distribution (Fig. 2A). Myosin-IIB is also polarized when cells are placed on stiff matrix where increased stress is applied to cells (Fig. 2B). These results thus show that myosin-IIB responds to external forces.
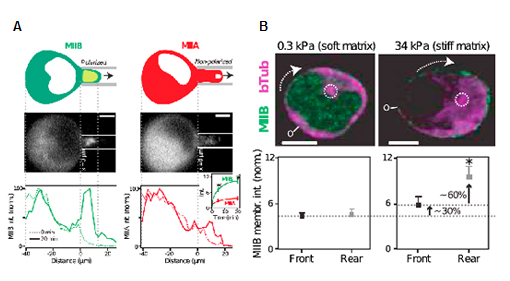
Figure 2. Polarization of myosin-IIB by externally applied forces. (A) Localization of myosin-IIB and IIA under negative fluid shear applied by micropipette aspiration. (B) Localization of myosin-IIB in HSPCs migrated on soft and stiff matrix.
3) Asymmetric division of HSPCs requires myosin-IIB-
After confirming that external forces polarize myosin-IIB, the direct role of myosin-IIB in asymmetric division was investigated. Consistent with the results in Fig. 1A, myosin-IIB is localized in daughter cells that are also CD34+, which is the marker for human HSPCs (Fig. 3A). Interestingly, siRNA against myosin-IIB increases the frequency of symmetrically dividing CD34+ cells ex vivo, suggesting HSPC expansion. The human stem cell expansion after myosin-IIB knockdown was functionally confirmed in vivo by using intrabone transplantation into a xenograft mouse model (Fig. 3B). As a result of stem cell expansion in the marrow compartment, donor-derived mature cells is decreased in peripheral blood cells.
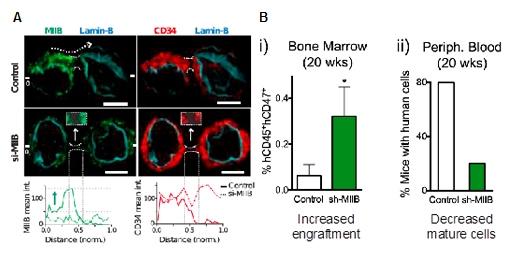
Figure 3. Myosin-IIB in asymmetric HSPC division. (A) Localization of myosin-IIB and CD34 in dividing HSPCs before and after RNAi against myosin-IIB. (B) Intrabone transplantation of human CD34+ cells transduced with scrambled or myosin-IIB shRNA in NOD/SCID/IL2r-/- (NSG) mice.
4) Myosin-IIA is required for sustained blood production-
As myosin-IIA expression is relatively constitutive among different blood cell types, its role in hematopoiesis in vivo was tested by competitive bone marrow transplantation (Fig. 4A). Conditional deletion of MYH9 gene leads to progressive decrease in both myeloid and lymphoid lineages in peripheral blood derived from donor cells (Fig. 4B). A compensatory increase in competitor cells was observed. Interestingly, the rate of decrease in donor peripheral blood cells is consistent with the known natural decay rate of each blood lineage. This indicates that myosin-IIA per se does not lead to cell death in differentiated cells, but leads to defective blood cell production from the stem cell level.
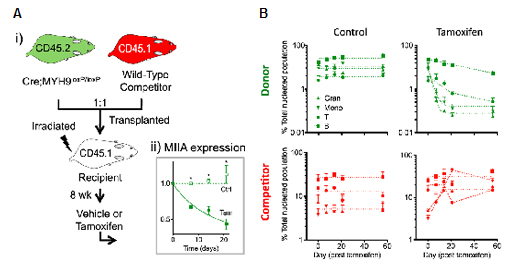
Figure 4. Myosin-IIA in blood production. (A) Bone marrow cells from conditional myosin-II knockout mice (donor) were mixed 1:1 with those from wild-type mice (competitor), and transplanted into lethally irradiated recipients. After 8 weeks, the recipient mice were treated with either vehicle or tamoxifen to induce deletion of MYH9 gene specifically in donor cells. (B) Monitoring peripheral blood lineages upon tamoxifen treatment.
5) Transient inhibition of both myosin-II isoforms enriches for functional stem cells-
Inhibition of myosin-IIB and IIA decreases mature blood cells due to increased symmetric stem cell division and defective stem cell differentiation, respectively. This suggests that transient inhibition of both isoforms by drugs against myosin-II could be useful in enriching for the stem cell population with a minimal use of surface markers by eliminating more mature cells. Consistent with this prediction, blebbistatin increases the frequency of functional human stem cells by ~4 fold (Fig. 5A). Compared to untreated cells, CD34+ cells treated with blebbistatin shows a gene expression profile closer to freshly isolated stem cells using multiple surface markers (Fig. 5B). The results thus show the utility of pharmacological inhibition against myosin-II to enrich for functional stem cells.
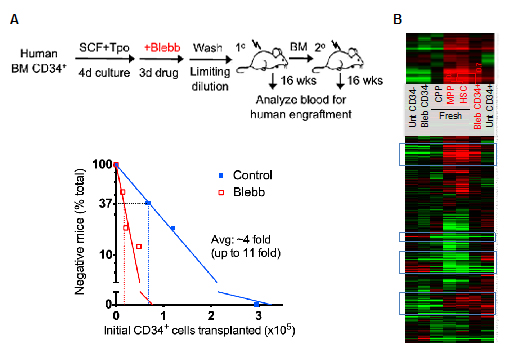
Figure 5. Enrichment of HSCs by myosin-II inhibition. (A) Human CD34+ cells were treated with blebbistatin ex vivo. Cells were then washed and transplanted into NSG mice in limiting dilution to quantify the frequenty of human blood repopulating cells. (B) Microarray profiling of HSPC populations from fresh and cultured cells.
- 4. Implications
-
The study is one of the first reports to show that physical forces regulate stem cell polarity and division symmetry. The results show that the B isoform of myosin-II is not only polarized in response to external forces but also required for asymmetric division. While pan-myosin-II inhibitors are useful for enriching stem cells, it will be interesting to develop a reversible inhibitor against the B isoform, and explore whether it will be useful to expand stem cells ex vivo and in vivo. Moving forward, it will be worthwhile to investigate whether myosin-II isoform expression and cellular contractility change during abnormal hematopoiesis.
- 5. About the author
-
This work was done in the Biophysical Engineering Laboratory of Dr. Dennis Discher at the University of Pennsylvania where Dr. Shin completed his Ph.D. He then worked as a postdoctoral fellow in Bioengineering at Harvard University in the laboratory of Dr. David Mooney. Dr. Shin recently joined the University of Illinois College of Medicine as an Assistant Professor of Pharmacology and Bioengineering. His laboratory is investigating how mechanical properties of stem cells can be leveraged to control their delivery, targeting, and efficacy in the body. To facilitate this investigation, his laboratory offers an interdisciplinary research environment at the interface between medical science and engineering. For further information, please visit: http://mcph.uic.edu/shin
